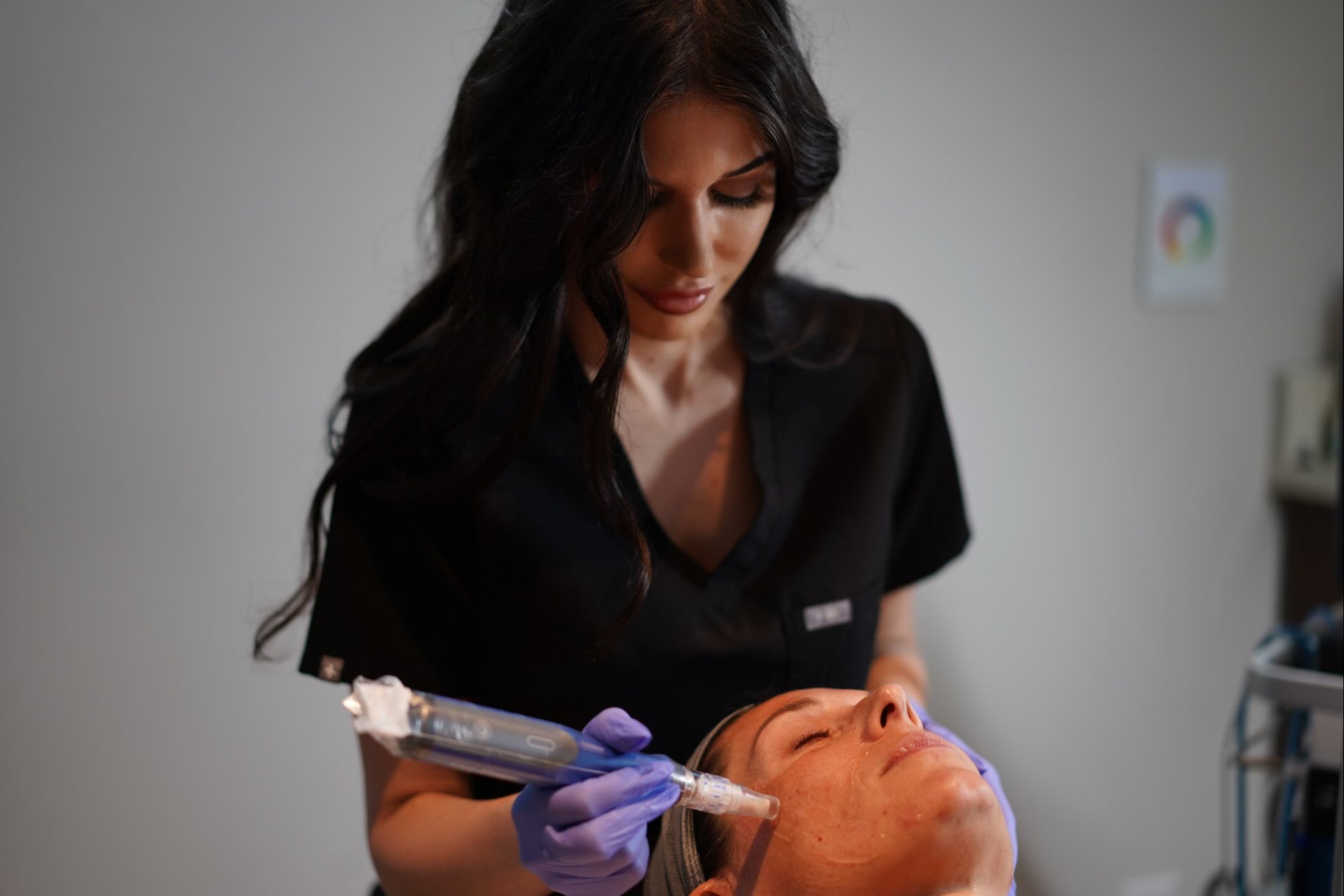
Microneedling with SkinPen
SkinPen creates controlled micro-injuries to stimulate the body’s natural wound-healing process, while minimizing cellular damage. SkinPen is a minimally invasive procedure performed in-office with little downtime.
Results Matter
FAQs
-
SkinPen by Bellus Medical is the first micro-needling device in the world cleared by the U.S. Food and Drug Administration (FDA), clinically shown to safely and effectively treat the appearance of skin. In fact, 90% of subjects in the clinical trial would recommend the treatment to friends and family.
-
SkinPen creates hundreds to thousands of “micro” skin punctures per second to stimulate the skin’s natural wound healing process – inflammation, proliferation, and remodeling – to prompt tissue remodeling without causing scar tissue formation. Most patients can return to normal activities within 24 hours.
-
SkinPen sets the industry standard for safety as the world’s first microneedling device cleared by the U.S. Food and Drug Administration. SkinPen has a patent-pending – and single-use – sterile needle cartridge and is surrounded by a propriety BioSheath that acts as a barrier to prevent cross-contamination between procedures.
-
SkinPen is clinically proven to fight the appearance of neck wrinkles and reduce the appearance of acne scars. In fact, 90% of subjects in the clinical trial would recommend the treatment to friends and family. It’s a minimally invasive procedure performed in-office with little to no downtime. As the first FDA-cleared microneedling device, SkinPen sets the industry standard for safety.
-
The skin will appear slightly pink to red immediately post-procedure, similar to a mild to moderate sunburn. Additionally, the most common treatment responses experienced were dryness, rough skin, tightness, redness, itching, peeling, discomfort, tenderness, and burning. These conditions resolved over time without any further complications.


